Industry
Overview
The pharmaceutical industry develops, produces, and markets drugs or pharmaceuticals licensed for use as medications. Pharmaceutical companies are allowed to deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations regarding the patenting, testing and ensuring safety and efficacy and marketing of drugs particularly prescription drugs. From its beginnings at the start of the 19th Century, the pharmaceutical industry is now one of the most successful and influential industries, attracting both praise and controversy, and its activity is subject of much market research.
The industry is worth more than $1 trillion in 2014, marking a 5% compound annual growth rate. The US is at the top of the global pharmaceutical market and is expected to hit almost $345 billion in 2014. The US is followed by the Japanese market, which is followed by the European market.
History
The earliest drug store date to the middle Ages. The first known drug store was opened by Arabian pharmacists in Baghdadin754, and many more soon began operating throughout the medieval Islamic world and eventually medieval Europe. By the 19th century, many of the drugstores in Europe and North America had eventually developed into larger pharmaceutical companies.
Most of today's major pharmaceutical companies were founded in the late 19th and early 20th centuries. Key discoveries of the 1920s and 1930s, such as insulin and penicillin, became mass-manufactured and distributed. Switzerland, Germany and Italy had particularly strong industries, with the United Kingdom, the United States, Belgium and the Netherlands following suit.
Legislation was enacted to test and approve drugs and to require appropriate labeling. Prescription and non-prescription drugs became legally distinguished from one another as the pharmaceutical industry matured. The industry got underway in earnest from the 1950s, due to the development of systematic scientific approaches, understanding of human biology and sophisticated manufacturing techniques. Numerous new drugs were developed during the 1950s and mass-produced and marketed through the 1960s.
Attempts were made to increase regulation and to limit financial links between companies and prescribing physicians, including by the relatively new U.S. Food and Drug Administration (FDA). In 1964, the World Medical Association issued its Declaration of Helsinki, which set standards for clinical research and demanded that subjects give their informed consent before enrolling in an experiment. Pharmaceutical companies became required to prove efficacy in clinical trials before marketing drugs.
The industry remained relatively small scale until the 1970s when it began to expand at a greater rate. Legislation allowing for strong patents, to cover both the process of manufacture and the specific products, came into force in most countries. By the mid-1980s, small biotechnology firms were struggling for survival, which led to the formation of mutually beneficial partnerships with large pharmaceutical companies and a host of corporate buyouts of the smaller firms. Pharmaceutical manufacturing became concentrated, with a few large companies holding a dominant position throughout the world and with a few companies producing medicines within each country.
The pharmaceutical industry entered the 1980s pressured by economics and a host of new regulations, both safety and environmental, but also transformed by new DNA chemistries and new technologies for analysis and computation. Drugs for heart disease and for AIDS were a feature of the 1980s, involving challenges to regulatory bodies and a faster approval process.
Managed care and Health maintenance organizations (HMOs) spread during the 1980s as part of an effort to contain rising medical costs, and the development of preventative and maintenance medications became more important. A new business atmosphere became institutionalized in the 1990s, characterized by mergers and takeovers, and by a dramatic increase in the use of contract research organizations for clinical development and even for basic R&D. The pharmaceutical industry confronted a new business climate and new regulations, born in part from dealing with world market forces and protests by activists in developing countries. Animal Rights activism was also a challenge.
Marketing changed dramatically in the 1990s. The Internet made possible the direct purchase of medicines by drug consumers and of raw materials by drug producers, transforming the nature of business. In the US, Direct-to-consumer advertising proliferated on radio and TV because of new FDA regulations in 1997 that liberalized requirements for the presentation of risks.
Drug development progressed from a hit-and-miss approach to rational drug discovery in both laboratory design and natural-product surveys. Demand for nutritional supplements and so-called alternative medicines created new opportunities and increased competition in the industry. Controversies emerged around adverse effects, notably regarding Vioxx in the US, and marketing tactics. Pharmaceutical companies became increasingly accused of disease mongering or over-medicalizing personal or social problems.
Pharmaceutical Innovation and Public Health
Drug discovery is the process by which potential drugs are discovered or designed. In the past most drugs have been discovered either by isolating the active ingredient from traditional remedies or by serendipitous discovery. Modern biotechnology often focuses on understanding the metabolic pathways related to a disease state or pathogen, and manipulating these pathways using molecular biology or biochemistry. A great deal of early-stage drug discovery has traditionally been carried out by universities and research institutions.
Drug development refers to activities undertaken after a compound is identified as a potential drug in order to establish its suitability as a medication. Objectives of drug development are to determine appropriate formulation and dosing, as well as to establish safety. Research in these areas generally includes a combination of in vitro studies, in vivo studies, and clinical trials. The amount of capital required for late stage development has made it a historical strength of the pharmaceutical companies.
Pharmaceutical Innovation & research The research-based pharmaceutical industry plays a unique role in developing new medicines and vaccines to prevent and treat diseases, and improve the lives of patients. Its key contribution to medical progress is turning fundamental research into innovative treatments. Industry’s success rests on continuous innovation – for the prevention and treatment of common, complex, and neglected diseases, and for improvements in existing treatments. Despite challenging business conditions, the industry undertakes investments that are considerably more risky than those in other high-technology sectors. By investing billions of dollars and thousands of scientist-hours, it pushes the limits of science, improves global health, and contributes to the prosperity of society.
For the past 100 years, the private sector has produced nearly all the medicines and vaccines on the market. When a pharmaceutical company invests in research and development (R&D) of new medicines and vaccines, it first screens for chemical and biological compounds that exhibit the potential for treating new or existing conditions. Accordingly, for any particular medicine, R&D begins once researchers identify a promising compound among the 5,000–10,000 screened, on average. Researchers then extensively test the compound to ensure its efficacy and safety, a process that can take 10 to 15 years.1 To illustrate, in 2011, 35 new medicines were launched, while more than 3,200 compounds were at different stages of development.2 The difference in these numbers indicates the many research hurdles to be overcome before compounds can be developed into safe and effective medicines.
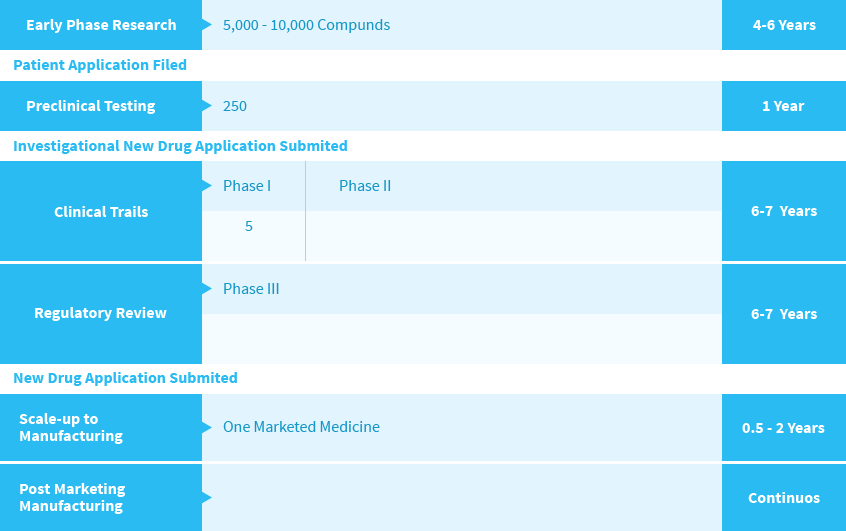
The Cost of Innovation Today, the cost of developing a single drug amounts to over USD 1.3 billion4compared to USD 138 million in 1975. This ten-fold increase reflects the varioustechnical, regulatory and economic challenges facing R&D pipelines. Companies oftenexperience lost R&D investments (that is, R&D expenditures that do not materialize ina market-approved medicine) because pharmaceutical R&D is marked by high failurerates. The research-based pharmaceutical industry is estimated to have spentnearly USD 135 billion globally on pharmaceutical R&D in 2011.
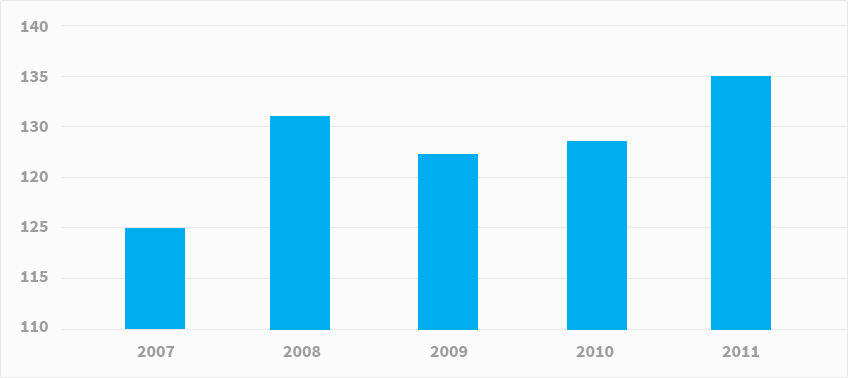
Pharmaceutical R&D spending (USD billion) Rising R&D costs and more stringent testing requirements have been accompanied by a decline in new medicine approvals. The number of new chemical or biological entities (NCE sand NBEs) launched on the world market fell to 149 in the 2007–2011 period compared with196 a decade earlier.
Access to medicines and healthcare systems
A robust healthcare system is an important pillar of the development process, and asound pharmaceuticals policy is a fundamental condition for health systems to performwell. Health systems are complex mechanisms through which health products, services,and care are delivered to patients. Their success requires joint effort and collaborationamong all the key health actors. As such, the research-based pharmaceutical industryplays an essential role in providing access to medicines and support to the overallhealthcare structure.
The world is still marked by a sharp disparity in the wealth of countries, which has a major impact on the performance of healthcare systems.On average, low-income countries spend 5.4% of GDP on financing healthcare systems whereas high-income countries spend more than 11 % on health. The disparities are also significant in terms of healthcare workers. These divergences in wealth and resources have a decisive impact on people’s health.
In terms of funding, performing healthcare systems require sufficient allocation of resources by government and/or the private sector. Unfortunately, public health and the strengthening of healthcare systems are not seen as important priorities in many countries, and the resources made available to health vary significantly from country to country.
Strong healthcare systems also require strategic long-term planning and political commitment. Health authorities should not only facilitate necessary resources, but also procure medicines effectively and minimize inefficiencies and unnecessary mark-ups in the supply chain, such as taxes and tariffs.
Economic footprint of the pharmaceutical industry
The research-based pharmaceutical industry makes a major contribution to theprosperity of the world economy. It is a robust sector that has been one of the pillars ofindustrialized economies and is increasingly recognized as an important sector in thedeveloping world as well. It contributes to employment (direct, indirect, or induced), trade (through imports and exports), expenditure on research and development (R&D), and technological capacity building. It is also a necessary foundation for the existence of the generic industry.
The pharmaceutical industry’s activities have a strong and positive influence onthe economy. This economic footprint is most visible in the form of investments inmanufacturing and R&D, but it often has other positive socioeconomic impacts, such as constant improvements in academic research. It also stimulates the creation of companies that support parts of the research and production process.
Pharmaceutical Industry Leaders
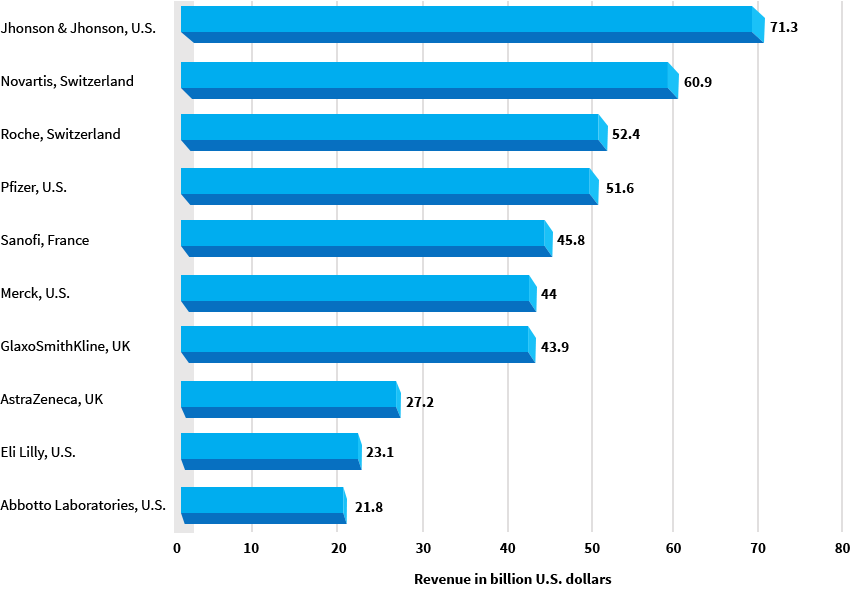
Industry analysts list the top industry leaders as Pfizer, Hoffmann-La Roche, Novartis, GlaxoSmithKline, Sanofi-Aventis, AstraZeneca, Abbott Laboratories, Merck & Co and Bristol-Myers Squibb.
Industry
Pharmaceutical innovations are behind some of the greatest achievements in modernmedicine. Today people live longer and healthier lives than previous generations.Medical advances allow people to enjoy a better quality of life and increase their productivity,contributing to the overall prosperity of society. Pharmaceutical industry also creates jobs, spurs technology, and represents an important source of income. Unfortunately,not everyone has yet fully benefited from these medical advances. Poverty and greatwealth inequality between and within countries mean that many do not have accessto even the simplest healthcare interventions. Addressing these issues is a complexchallenge that requires long-term commitment from government, civil society, and theprivate sector. Through differential pricing schemes, donation programs, and technologytransfer initiatives, the pharmaceutical industry has been doing its part to help thosein greatest need to also enjoy the benefits of medical progress. Much still needs tobe done; the path forward will require a constant rethinking on how to maximize theresearch-based industry’s positive impact on the health and prosperity of societies.
“We Value life by making several significant industry contributions in the development of the market, educating the public on health benefits and improving infrastructure, work practices and behaviors”.








